The AmDrop™ Baby Face Treatment is a unique combination of world-class biologics used in conjunction with many regenerative treatments in med spas and plastic surgery settings.
Product Description
Our kit is designed to provide Aesthetic practitioners with a carefully selected array of components which facilitate fast and complete recovery following many aesthetic procedures including micro-needling, scar removal, and laser skin resurfacing.
The AmDrop™ Baby Face Treatment is a unique combination of world-class biologics used in conjunction with many regenerative treatments in med spas and plastic surgery settings.
The AmDrop™ Baby Face Treatment includes our AmDrop™ pure liquid allograft fluid, a professional grade hydra-needle by Integrity, Hyaluronic Acid, and a unique 3D Hyaluronic Acid Mask.
Use Cases
AmDrop™ Baby Face Treatment can be used in a wide array of cases such as:
- Microneedling
- Facial Rejuvenation
- Post Laser Skin Resurfacing Treatments
- Hair Restoration
- Non-Surgical Face lift
Additional Information
AmDrop™ products are primarily amniotic liquid allografts derived from the amniotic components of the placenta to advance soft tissue repair, replacement, and reconstruction. It is classified as minimally manipulated under FDA regulation 21 CFR Part 1271 and section 361 of the PHS. Amnion-derived fluid and membranes are rich in the essential components necessary for tissue regeneration and repair including:
- Growth Factors
- Cytokines
- Collagen
- Hyalauronic Acid
- Mesenchymal components
How it Works
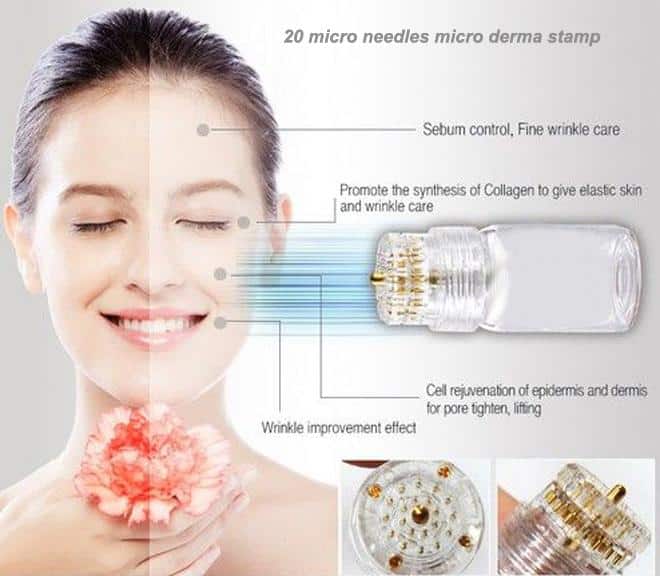
We strive to help medical professionals improve their patients’ quality of life by leveraging the latest biologic therapies to provide advanced care for what would otherwise be non-treatable conditions.
Ready to Use
AmDrop™ amniotic fluids are delivered in sterile vials as ready-to-use, flowable grafts; no thawing or additional preparation is required.
Stable shelf life
AmDrop™ is stored at room temperature (50-86°F/10-30°C) until ready for use. Product has a one-year shelf life.
Immunosuppressive
AmDrop™ has been shown to suppress the immune response and modulate inflammation.
Sterile
AmDrop™ is provided in a sterile vial in a clear peel-pouch and may be introduced to the sterile field.
Safety
Human tissue donors undergo a stringent quality assurance review process. Additionally, donor blood testing and screening verifies samples are negative or nonreactive for the following communicable disease markers:
- HUMAN IMMUNODEFICIENCY VIRUS (HIV): HIV-1/2 Antibodies, Nucleic Acid Test for HIV-1 RNA
- HEPATITIS B VIRUS (HBV): HBV Surface Antigen, HBV Core Antibody (Total), Nucleic Acid Test for HBV DNA
- HEPATITIS C VIRUS (HCV): HCV Antibody, Nucleic Acid Test for HCV RNA
- HUMAN T CELL LYMPHOTROPHIC VIRUS I/II: HTLV-I/II Antibody
- SYPHILIS: Rapid Plasma Reagin Screen, Treponemal Specific Test
- WEST NILE VIRUS: Nucleic Acid Test for WNV RNA
