Progress Medical is doing our part to help navigate through these trying times in dealing with the COVID-19 Virus. Testing can be implemented for any Organization or School utilizing a healthcare provider and the software solution. Below is guidance for a back to work plan.
Progress Medical now offers a complete COVID-19 Testing Solution combining Rapid Antibody, Detection via Rapid Antigen and PCR Saliva Testing with software management
Assure FDA EUA COVID-19 IgG/IgM Rapid Antibody Test
The COVID-19 IgG/IgM Rapid Test Device is an in vitro immunoassay for the direct and qualitative detection of anti-SARS-CoV-2 and
anti-SARS-CoV-2 IgG in human whole blood, serum or plasma. This is the first antibody test to receive authorization for point of care utilizing a fingerstick.
- Results in 15 minutes
- Easy to use
- No equipment required
- High accuracy
Combined Sensitivity 100%
Combined Specificity 98.8%
FDA EUA BD Veritor System for Rapid Detection of SARS-CoV-2
When you need fast, reliable SARS-CoV-2 testing, turn to the BD Veritor Plus System. This offers lab quality results at the point of care, in a simple-to-operate, handheld instrument.
- Simplify the testing process
- Achieve fast, reliable results
- Provide workflow efficiency
- Provide result traceability
Infinity BiologiX FDA EUA Qualitative Detection with Saliva (PCR)
- Saliva sample determines active infection of SARS-CoV-2 virus
- First FDA EUA Saliva test approved for an at-home saliva sample collection
- Secure digital lab results with 72 hours of receiving the sample
- Replaces uncomfortable Nasal Swab test
- Spit into a tester and mail to the lab
- High accuracy
COVID-19 SOFTWARE SOLUTION utilized with PCR Saliva Test
Test Results, Patient Education, & Compliance Software
With each test, we offer a Patient Communication, Patient Education, & Health Assessment Surveys regarding COVID-19 to healthcare providers at no cost.
This automated tool allows providers to effectively communicate with their patients:
- Accurate CDC Patient Education COVID Health Assesment Survey (CVDHA)
- Health Assesment Tracking & Results PROMIS Global Health Survey
- Quarantine Guidance
- Ability to include other custom surveys
The COVID Health Assessment Survey (CVDHA) will be sent automatically to patients within an educational Care Pathway and then resent at a cadence of 1, 2, 3, 4, and 6 weeks for continued risk assessment.
The HIPAA-Secure platform enables patients to access accurate, reliable information from a provider they trust and safely increase patient-provider communication. It also provides a way for healthcare providers to track patient symptoms, evaluate health risks, and encourage informed patient engagement.

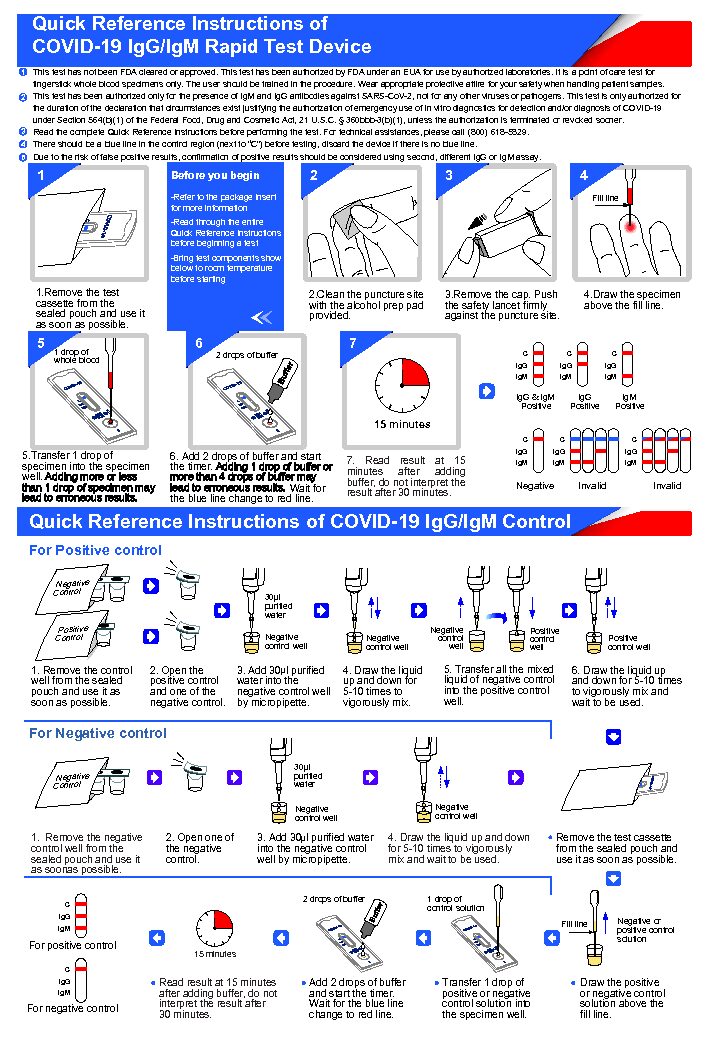

This test is authorized for use at the Point of Care (POC), i.e. in-patient care setting under a CLIA. Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Progress Medical can provide guidance on how to receive a Certificate of Waiver in under two weeks, should you need one.
The following tests can be offered with the BD Veritor System:
BD Veritor System COVID Tests 30 tests/kit
Influenza A+B POC Kit, CLIA Waived, 30 tests/kit
RSV Test Kit, CLIA Waived, 30 tests/kit
Strep A Test Kit, CLIA Waived, 30 tests
How does this test work?
This is a polymerase chain reaction (PCR) test. It is a relatively simple and widely used molecular biology technique to amplify and detect DNA and RNA sequences and takes only a few hours. The test detects the expression of three genes specific to the SARS-CoV-2 virus, the N, S, and ORF1ab genes. Viral RNA is extracted from the patient’s saliva sample and the presence of gene sequences is assessed. If the presence of 2 or 3 of these gene transcripts is confirmed, the test is considered POSITIVE.
How accurate is this test?
The assay can detect the virus down to fewer than 10 copies per milliliter of saliva, which is highly sensitive. In practice, 98% of test results are either positive or negative, with only 2% being inconclusive. The sequences that help to detect the virus (primers) match the viral sequences almost 100% of the time, which ensures that the test is specific only to the 3 selected viral genes, which in combination are only found in the SARS-CoV-2 virus.
